 Kirthana Balachandran
Kirthana BalachandranThe first therapy that uses gene-editing is to be offered on the NHS in a “revolutionary breakthrough” for patients.
It will be used as a potential cure for the blood disorder beta thalassaemia.
Stem cells which make blood will be extracted, reprogrammed to correct the condition and returned to the patient’s body.
It could spare them needing a blood transfusion, every three-to-five weeks, for life.
People with beta thalassaemia struggle to produce enough haemoglobin, which is the protein in red blood cells that carries oxygen around the body.
It is a genetic disease that is passed down through families and caused by defects in the body’s instructions for manufacturing haemoglobin.
It can leave people severely tired, weak, and short of breath and also cuts life expectancy.
Kirthana Balachandran, 21, was diagnosed when she was only three months old and gets muscle pain and back pain and can have palpitations when walking uphill.
“The idea of depending on transfusions for quite literally the rest of my life is daunting, I constantly worry about the future,” she says.
The idea of gene-editing is you only need to do it once.
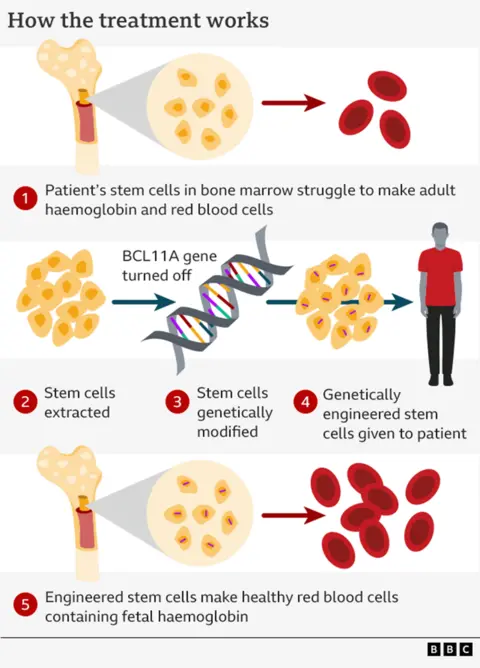
How the gene-editing works
It relies on the a tool called Crispr, which won the Nobel Prize for Chemistry in 2020. It is essentially a satnav connected to a pair of scissors – one part targets the right section of DNA and the other performs the edit.
You might think this technology is being used to repair the genetic defect, but it is actually cuter than that.
Instead it relies on the fact your body makes different types of haemoglobin before and after birth.
When we are still in the womb, our bodies use “fetal haemoglobin” to pull oxygen out of our mother’s bloodstream.
Once we are born, a genetic switch is flipped and we start making “adult haemoglobin”.
Crucially, it is only the adult form of haemoglobin that is affected by beta thalassaemia.
So the therapy disables the switch – named BCL11A – so the adult body starts making fetal haemoglobin once again.
In order to do this, the stem cells that manufacture red blood cells in our spongy bone marrow are harvested.
These cells are sent to the lab where the genetic switch is targeted.
The next stage is unpleasant. It takes a course of chemotherapy to kill off the old stem cells that were producing broken haemoglobin, before the new ones can be put in.
Abdul-Qadeer Akhtar, 28 and from Hemel Hempstead, took part in clinical trials in 2020.
He said the treatment was “challenging” and “tough” but since having it, he has been “healthier and more active”.
“[I] have even taken up boxing, I can travel more freely now, which is fantastic, I’m eager to embrace life to the fullest.”
Data shows that out of 52 patients given the treatment, 49 did not need another blood transfusion for at least a year of monitoring. The approach is so new that the long-term effects are still unknown.
Kirthana says: “With gene therapy, I would potentially have a cure, and not have to have my three-weekly transfusions.
“It would be a life-changing treatment.”
A £1.6m price tag?
The National Institute of Health and Care Excellence medicines watchdog has approved the therapy after assessing the costs and benefits of what it calls a “potential cure”.
A deal has been struck which means NHS England is paying less than the official price of £1.6m per patient.
It is estimated that 460 people over the age of 12 would be eligible if they wanted the therapy, and it will be offered at seven specialist centres “within weeks”.
Amanda Pritchard, the NHS chief executive, said: “This is a historic moment for people living with beta thalassaemia with a potential cure for those facing this debilitating disorder now available on the NHS.”
Beta thalassemia mainly affects people of Mediterranean, south Asian, south-east Asian and Middle Eastern backgrounds.
Previously the only alternative to blood transfusions was a stem cell transplant, but this was rare because it required a very close tissue match from a donor.
Casgevy, developed by the company Vertex, is the first approved therapy to use Crispr-technology.
Romaine Maharaj, executive director of the UK Thalassaemia Society, said “we stand on the brink of a revolutionary breakthrough” and “it is a beacon of hope”.
Negotiations are still under way to see if the same therapy can be used on the NHS for another genetic disease affecting haemoglobin – sickle cell anaemia.


