You’re reading the web edition of STAT’s Health Tech newsletter, our guide to how technology is transforming the life sciences. Sign up to get it delivered in your inbox every Tuesday and Thursday.
Are health AI businesses zooming?
In a new report, Bessemer Venture Partners unpacks early insights about the prospects for artificial intelligence software services businesses it sees as a new category in the broader health tech ecosystem. This category includes AI clinical scribes, claims auditing tools, and back-office automation technology. BVP has observed a quick trajectory to $10 million in annual, recurring revenue — a metric that investors view as a crucial predictor of a business’ health. It takes AI companies 2.5 years to get to that $10 million benchmark compared to six years for health care software as a service players and three years for tech-enabled clinical services companies. The data is based on a cohort of 20 AI companies.
BVP attributes the speedy start to fast sales cycles driven by “urgency from buyers to test and buy AI-enabled solutions.” But! It’s careful to note that early revenue is often driven by trial programs that may not continue long term. (BVP, of course, is not an independent party given it invests in many of these companies.)
General Catalyst announces $750 million for health care as part of new fund
This morning, venture capital giant General Catalyst announced $8 billion in new capital, including $750 million dedicated to “health assurance.”
GC has made a number of aggressive bets in health care recently, including a bid to acquire Ohio-based Summa Health as a proving ground for innovative ideas. Notable portfolio companies today include Commure, Transcarent, and Hippocratic. Before digital health was an established category, GC was a prominent investor in diabetes management company Livongo, whose $18 billion sale to Teladoc is one of the the largest health tech exits to date.
Why are Change Healthcare breach notifications taking so long?
It’s October, and some people are only just now receiving notification that their sensitive data was compromised in the Change Healthcare ransomware attack that happened in February. By law, people are supposed to be notified within 60 days that their data was breached. So, what gives?
As STAT’s Brittany Trang reports, it’s complicated. In part, it’s unclear when exactly that 60-day clock is supposed to start. Moreover, Change is not a health care provider but a third party which processes insurance claims and helps facilitate insurance approvals and payments. In legal terms, it is a “business associate” of the companies doing the healthcare, and their responsibility is to inform their customer — a doctor’s office or a hospital — not the person whose data was compromised. You might notice your breach notification came on a Change Healthcare letterhead; that’s because Change volunteered to take responsibility for the process.
On Wednesday, Change parent UnitedHealth Group confirmed that it updated the number affected by the Change Healthcare cyberattack to 100 million people. “We continue to notify potentially impacted individuals as quickly as possible, on a rolling basis, given the volume and complexity of the data involved and the investigation is still in its final stages,” a spokesperson repeated to STAT.
Admittedly, that’s a lot of people to notify, but the fumbling of the breach notifications underscores what many observers see as the sorry state of health care cybersecurity. In fact, just last month Sens. Ron Wyden (D-Ore.) and Mark Warner (D-Va.) introduced a bill that would impose stricter cybersecurity rules on health care organizations and remove a cap on how much violators can be fined. UnitedHealth Group made $22 billion in profit last year. The thinking is, if you want to change cybersecurity practices, you’ve gotta hit them where it hurts. Read more here
Retina implant shows promise in people with severe vision loss
 Science Corporation
Science Corporation
Preliminary clinical trial data show that a retina implant restored vision for people whose central visual field has holes or blurry spots, Science Corporation reported this week. Trial participants could read text and recognize playing cards when using the implant, even though they were legally blind. The results, which have not been published in a peer-reviewed publication, are promising for millions of people who suffer from age-related macular degeneration, a leading cause of vision loss.
Called the Prima System, the implant relies on a camera mounted on glasses and a pocketable computer. The camera gathers infrared light and beams it to the implant that stimulates the retina.
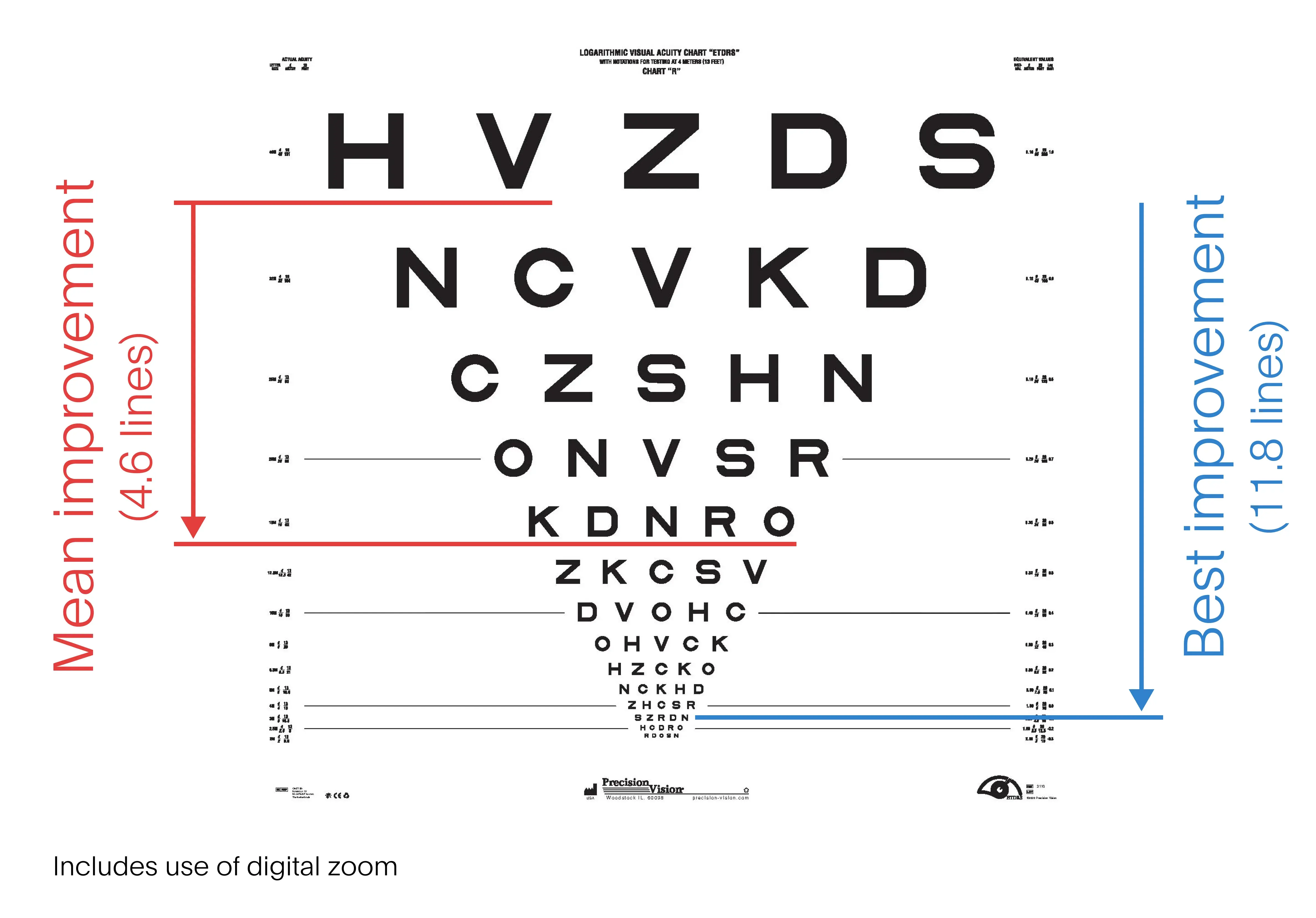
Science Corporation enrolled 38 people in the study, six of whom dropped out prior to testing. As STAT’s Timmy Broderick reports, after twelve months of use, the participants could read, on average, nearly five more lines, or 23 letters down an eye chart. That’s equivalent to a person’s eyesight improving from 20/320 to 20/200, which is the threshold for blindness in the United States. Read more here
Walmart’s pharmacy delivery push and a big venture hire
There was a lot of noise at HLTH this week but two developments caught my eye:
- Walmart announced that it will offer same-day pharmacy delivery in 49 states by the end of January. The offering is already live in six states. Walmart’s news follows Amazon‘s recent expansion of same-day pharmacy delivery.
- Early-stage venture capital firm Define Ventures added former Humana CEO Bruce Broussard as a partner. Big get!
AMA panel approves long-sought remote monitoring update
The American Medical Association‘s CPT Editorial Board, which is responsible for developing billing codes used by doctors, announced that it accepted proposed updates to remote monitoring codes that will take effect in 2026. While the exact details have not been released, the update will make it possible for doctors to bill for supplying devices in instances when fewer than 16 days of data are collected as well as to bill for fewer than 20 minutes of management services related to the monitoring. The effort to update the codes has taken several years with a few abandoned efforts along the way.
FDA’s new device chief and other agency notes
This week, the Food and Drug Administration confirmed what many people probably already suspected: Michelle Tarver will be the new director of the Center for Devices and Radiological Health, which oversees medical devices and hot-button topics like artificial intelligence tools and lab developed tests. Tarver joined the agency in 2009 and has been serving as the center’s interim chief since Jeff Shuren announced his departure over the summer.
- FDA also released new information about the upcoming inaugural meeting of the Digital Health Advisory Committee on November 20 and 21. At the public meeting, which will be streamed live on YouTube, the new committee will discuss “Total Product Lifecycle Considerations for Generative Artificial Intelligence-Enabled Medical Devices.” Comments for the meeting’s docket must be submitted by November 1st.
- Speaking at a conference for the biosimilars and generic drug industry, FDA chief Robert Califf hinted that the agency will use artificial intelligence tools to detect “cheating” in clinical trials, Bloomberg Law reports.
What we’re reading
- What’s on the docket for Congress post-election: Chinese biotech, Medicare payments, ACA subsidies, STAT
- Artificial intelligence–assisted colonoscopy for polyp detection: A systematic review and meta-analysis, Annals of Internal Medicine
- Performance of multimodal artificial intelligence chatbots evaluated on clinical oncology cases, JAMA Network Open

